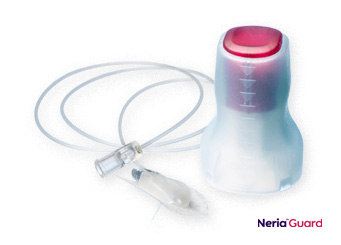
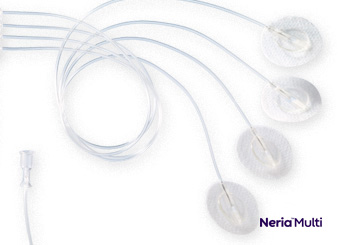
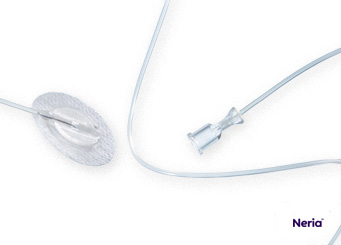
Soft Cannula Infusion Sets
Steel Needle Infusion Sets
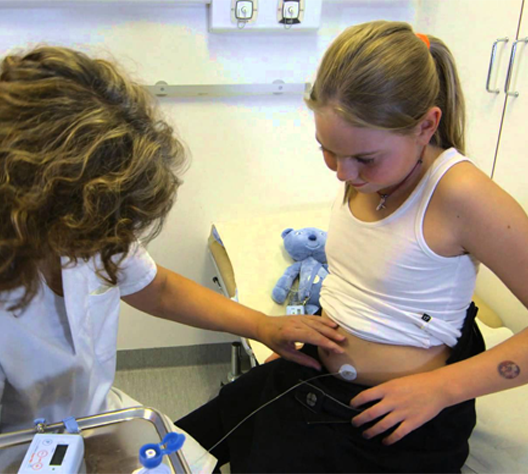
PAIN MANAGEMENT
Infusion Therapy
Best practice in pain management is to use the least invasive route of administration for the prescribed opioid. As a consequence patients are in most cases introduced to opioid pain management in a short acting formulation by taking the morphine in an oral form.1 However, if the pain continues and relief is not reached, or in cases of severe pain, patients may be prescribed stronger opioids by oral administration. If patients are unable to take medication orally, then opioids may be administered by injection or continuous infusion. Subcutaneous infusion of opioids is considered preferable, as it is less invasive and may be better tolerated than intravenous infusion, and less painful than intramuscular injection.
neria™ infusion sets for Pain Management
As relief and comfort are areas of focus in pain management, especially in palliative care, the soft cannula range of neria infusion sets are recommended in many cases for the infusion. These infusion sets have a disconnect feature which make the sets convenient to use during long infusion periods.
In general, however, the choice of infusion set should always be based on the health care professional’s assessment of each individual patient’s situation.
Clinical studies
Clinical studies showed that neria™ infusion sets helped minimize pain during insertion and reduced needle related traumas.4,5
neria™ infusion sets have been tested for safe use with hydromorphone, morphine sulfate and morphine chloride for pain management 2,3
- WHO web site, www.who.int/cancer/palliative/painladder/en/
- Drug Compatibility Study of Three Infusion Devices with Morphine Sulfate, version 1.4, IKFE 10006, September 2, 2011, Data on File.
- Drug Compatibility Study of Three Infusion Devices with Hydromorphone Hydrochloride, version 1.4, IKFE 10006, September 2, 2011, Data on File.
- Chan G.C.F, Ng D.M.W. et al, Comparison of Subcutaneous Infusion Needles for Transfusion-Dependent Thalassemia Patients by the Intrapersonal Cross-Over Assessment Model, Am.J.Hematol.76;398-404 (2004).
- Parsons J. Infusion line preferences of patients prescribed apomorphine for complex Parkinson’s disease. British Journal of Neuroscience Nursing. December 2009.Vol 5 No 12.
PRIMARY IMMUNE DEFICIENCY
What is Primary Immune Deficiency?
The human immune system protects the body against infections. Immune deficiency disease occurs when part of the immune system does not function correctly or is absent.
Patients with primary immune deficiency lack immunoglobulins (antibodies), which are special proteins in the blood that fight infections and are important for a well-functioning immune system. Patients suffering from a primary immune deficiency often have a diminished quality of life as their susceptibility to infection increases significantly. However, with proper medical care, many patients live full and independent lives.
Immunoglobulin replacement therapy as treatment for primary immune deficiency
Immunoglobulin therapy is one of several effective medical therapies available to treat primary immune deficiency. To replace the missing immunoglobulins, extra immunoglobulins are prepared from the blood plasma from healthy donors and injected into the patient’s blood by infusion, administered subcutaneously or intravenously. This is replacement therapy and is necessary for the rest of the patient’s life.
Treatment frequency
Treatment frequency depends on the route of administration and the type of drug. The patient uses a mechanical or a battery-driven, syringe driver infusion pump to deliver a specified continuous amount of immunoglobulins over a self-administered period – either subcutaneously or intravenously.
Subcutaneous infusion of immunoglobulins with neria™ infusion sets
When subcutaneous infusion is preferred, neria™ infusion sets are the perfect option. The patient inserts an infusion set under the skin and connects it to the infusion pump. neria™ infusion sets are particularly well suited for the delivery of immunoglobulins, with a choice of multi-site infusion sets for larger volumes.
neria™ infusion sets are tested (in vitro) for use with immunoglobulin therapy1. The quality and careful design of neria™ infusion sets make them an excellent choice for patients treated with immunoglobulins.
Clinical studies
Clinical studies showed that neria™ infusion sets helped minimize pain during insertion and reduced needle related traumas.2,3
- Compatibility and stability testing of the catheter device Unomedical comfort™ with the immunoglobulin Subcuvia. IKFE 09016. April 16, 2009. Data on file.
- Chan G.C.F, Ng D.M.W. et al, Comparison of Subcutaneous Infusion Needles for Transfusion-Dependent Thalassemia Patients by the Intrapersonal Cross-Over Assessment Model, Am.J.Hematol.76;398-404 (2004).
- Parsons J. Infusion line preferences of patients prescribed apomorphine for complex Parkinson’s disease. British Journal of Neuroscience Nursing. December 2009.Vol 5 No 12
The neria™ infusion set is a specialised infusion set developed specifically for subcutaneous drug delivery, including immunoglobulins for primary immune deficiencies, deferal for thalassaemia (a rare blood disorder), morphine for pain management and apomorphine for Parkinson’s disease. Infusion sets for these therapeutic areas are distributed under our brand name neria™.
The neria™ infusion sets can be used in different therapy areas and provide a number of features which support the use of the products for both patients and healthcare professionals:
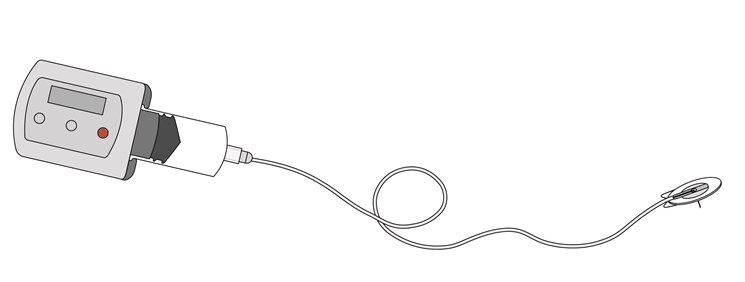
- Simplicity
Simple and easy-to-use features such as pre-attached adhesive - Comfort
Soft and flexible cannulae options and visual monitoring of sites - Safety
In vitro tested especially for subcutaneous use and drug compatible1,2,3,4,5 - Efficacy
Strong double layer tubing with very low priming volumes
Clinical studies have shown that neria™ infusion sets helped minimize pain during insertion and reduced needle related traumas.6,7
The neria™ Infusion Set Overview
| Product Specifications | neria™ | neria™ multi | neria™ guard |
| Cannula | Stainless Steel | Stainless Steel | Soft |
| Insertion angle | 90 degrees | 90 degrees | 90 degrees |
| Disconnection feature | No | No | Yes at site |
| Needle / Introducer needle gauge | G27 or G29 | G27 | G27 |
| Needle lengths | 6-8-10-12 mm | Bi-furcated: 8-10mm Tri-furc: 8-10-12mm Quad-fur: 8-10-12mm |
6 or 9 mm |
| Tubing lengths | 60-80-110 cm | Total 90 cm | 12, 60, 80, 110 cm |
| Adhesive | Built-in | Built-in | Built-in |
| Needle safety | – | – | Yes |
| Priming volumes | 60 cm @ 0.10 ml 80 cm @ 0.12 ml 110 cm @ 0.15 ml |
Bi-furcated ~ 0.36 ml Tri-furc ~ 0.43 ml Quad-furc. ~ 0.51 ml |
12 cm @ 0.04 ml 60 cm @ 0.10 ml 80 cm @ 0.12 ml 110 cm @ 0.15 ml |
Manage your Medication, Don’t Let it Manage You!
Discover our different solutions to help you control your medication for your different treatments.
INFUSION THERAPY
Therapeutic areas for subcutaneous infusion with neria™ infusion sets
neria™ infusion sets are subcutaneous infusion devices in vitro tested and approved for drug infusion in the treatment of Parkinson’s disease, thalassaemia and primary immune deficiency and pain management4,5
Read more about neria™ infusion sets here.

Subcutaneous drug delivery
Subcutaneous infusion delivers medication over a longer period in the fatty tissue beneath the skin, where the medication is absorbed by the body. A needle penetrates the skin, leaving either the needle or a soft catheter to remain in the tissue, from a few hours up to 72 hours. Needles and cannulae can vary in length, size, features and method of insertion. Infusion sets eliminate the need for one or several daily injections of a specific drug.

THALASSAEMIA
What is thalassaemia?
Thalassaemia is a genetic blood disorder whereby the body makes an abnormal form of hemoglobin – the protein in red blood cells that carries oxygen throughout the body. Without enough normal, healthy red blood cells, oxygen cannot reach all parts of the body and severe anemia occurs.
Complications resulting from thalassaemia
Some patients diagnosed with thalassaemia require regular blood transfusions to raise their hemoglobin blood levels. With each unit of blood transfused, an amount of iron is carried with hemoglobin, leading to iron build-up in the body’s tissue and organs also referred to as iron overload. As iron can be toxic, too much of it can damage tissue. When excessive iron accumulates in the heart, liver, lungs, brain, and other organs, the risk of disease, and even life-threatening conditions, is high.
Treatment of thalassaemia with desferal
Thalassaemia patients experiencing iron overload resulting from frequent blood transfusions require an iron removal or chelation therapy. One medication approved for this is desferal. When using an infusion pump, desferal is given over a 8-12 hour period, often overnight. The treatment can be performed daily, or 5-7 times a week.*
* According to Desferal Summary of Product Characteristics (SPC) by Novartis Pharmaceuticals UK Ltd.
Continuous subcutaneous infusion with desferal and neria™ infusion sets
The patient uses an elastomeric pump or a battery-driven, syringe driver infusion pump to deliver a specified continuous amount of desferal subcutaneously over a self-administered period.
The infusion sets should be changed with every treatment. The patient inserts an infusion set under the skin and connects it to the infusion pump.
neria™ infusion sets are tested (in vitro) for use with desferal.1 The quality and careful design of neria™ infusion sets make them an excellent choice for patients treated with continuous Desferal.
Clinical studies
Clinical studies showed that neria™ infusion sets helped minimize pain during insertion and reduced needle related traumas.2,3
- Drug Device Stability Test Thalaset – Study Desferal (deferoxamine mesilate). July 1, 2005. Data on file.
- Chan G.C.F, Ng D.M.W. et al, Comparison of Subcutaneous Infusion Needles for Transfusion-Dependent Thalassemia Patients by the Intrapersonal Cross-Over Assessment Model, Am.J.Hematol.76;398-404 (2004).
- Parsons J. Infusion line preferences of patients prescribed apomorphine for complex Parkinson’s disease. British Journal of Neuroscience Nursing. December 2009.Vol 5 No 12
Manage your Medication, Don’t Let it Manage You!
Discover our different solutions to help you control your medication for your different treatments.